Research Article / Open Access
DOI: 10.31488/HEPH.170
Betaglucan 0.2% versus Imiquimod 5% in the Treatment of Ano Genital Warts (Agw) Randomized Triple Blind Study 102 Patients Aged 18 to 50 Years Old in Managua, Nicaragua, Period June to September 2019 "(Preliminary Report- Due to the COVID 19 Epidemic the Cclinical Trial was stopped on August 2019. Registered in ClinicalTrials.gov with NCT 03901690 and included in the Clinical Trials Panel of the World Health Organization since November 2020
Dr. Alejandro Pérez Fabbri1, Álvaro Apestegui Barzuna2, Professor Romain Fantin3
1. Fellow, American College of Obstetricians and Gynecologists (FACOG) and Expert of the Federación Latino, Americana de Climaterio y Menopausia (FLASCYM- Latin American Federation of Climacteric and Menopause, Nicaragua
2. PhD in Biochemistry and clinical investigator, Nicaragua
3. MSc. Professional in Statistical Sciences and Information Analysis, Nicaragua
*Corresponding author:Dr. Alejandro Perez Fabbri, CEO CIMIF.
Abstract
Aim: The aim of the study was to evaluate the effectiveness of the soluble beta-glucan gel 0.2% in 102 women and men with ano-genital warts (AGWs) comparing with the actual gold standard treatment imiquimod cream 5%, the participants should have PCR or Hybrid Captation type 2 test performed and to develop AGWs clinically observed.
This is a triple blind study where the medical team ignores which patients are in intervention group branch A or in control group branch B. Only Professor Fanti,n Statistical Sciences has the control. Methods: From June 2019 to August 2019, when the clinical trial was stopped due to sanitary emergency created by Covid 19 epidemic. We have 36 participants; 20 men and 16 women, with an average age of 26 years old. In branch “A” we have 20 participants and in branch “B” have 16 participants; were recruited at the Saint Dominic Clinic, Managua, Nicaragua. The entire branch A participants were treated one cycle of a daily topical application of soluble beta-glucan gel 0.2% for five consecutive days with a suspension of 20 days. Until their next medical appointment of control in 3 weeks. All the branch B participants were treated one cycle of daily topical application of imiquimod cream 5% for three alternate days with a suspension of 20 days until their next medical appointment of control in 3 weeks. The effects of beta-glucan and imiquimod were analyzed clinically inspection and Pap smear control in women. The clinical trial protocol was analyzed, reviewed and approved by the Center of Investigations and Clinical Essays of the National Autonomous University of Nicaragua, (Spanish: Universidad Nacional Autónoma de Nicaragua, UNAN). Results: After 1 months of treatment, of the 36 participants’ women and men observed a Clearance rate of 80% in branch A with betaglucanes and Clearance rate of 50% in branch B with imiquimod. More than 70% of participants have had at least 5 sexual partners and practice 90% oral sex. Pink or gray genital warts are of greater therapeutic response with beta-glucan gel, especially those located on the glans penis and vagina. Only with the 35% of the sample it’s not enough data base to make conclusions. As CIMIF researchers’ team we are planning appropriate post covid epidemic conditions to continue the clinical trial.
Keywords: Ano-genital warts (AGWs), soluble betaglucanes, imiquimod, triple blind study
Introduction
Ano-genital warts (AGWs) or condylomata are caused by low-risk HPV and constitute the most prevalent sexually transmitted infection (STIs) worldwide [1]. The World Health Organization (WHO) estimates that the prevalence of HPV infection is between nine and thirteen percent or about 630 million people [2]. Most sexually active persons will have detectable HPV at least once in their lifetime.
Cervical Cancer
Each year there are more than half a million new cases and more than 300,000 women die due cervical cancer related to high-risk HPV, with a high prevalence, up to 75%, in the countries of medium and low income [8]. According to the new estimates of the Catalan Oncological Institute, the global prevalence of high-risk HPV, which was 10.2% in 2010, is currently 13%, which means 600 million people with HPV [11]. The prevalence by geographic regions is divided as follows: in Africa 22.4%, in America 13%, in Europe 8.2% and 7.9% in Asia [11]. According to the Madrid-based Institute Palacios de la Salud, [12] it estimates that in the European Community there are 195 million women over 15 years of age, of which: a) 15.5 million women are carriers of HPV. b) 2 million women with condylomata acuminata (CA) c) 2 million with low-grade squamous epithelial lesions (LSIL) d) 95,000 women wit d) 95,000 women with high-grade squamous epithelial lesions (HSIL) e) 33,000 new cases of invasive carcinoma. Therefore, it can be estimated that 20 million women over the age of 15 out of 195 million registered in the European Union (10.3% of the population in this age group), have a genital, clinical or subclinical condition at some point in their lives, attributable to HPV infections or any of its neoplasms.
It is estimated that 85 to 205 cases of genital warts per 100,000 people occur annually. The incidence is higher in younger adults and in women. The overall (females and males combined) reported annual incidence of any AGWs (including new and recurrent) ranged from 160 to 289 per 100,000, with a median of 194.5 per 100,000. New AGW incidence rates among males ranged from 103 to 168 per 100,000, with a median of 137 per 100,000 and among females from 76 to 191 per 100,000, with a median of 120.5 per 100,000 per annum. The reported incidence of recurrent AGWs was as high as 110 per 100,000 among females and 163 per 100,000 among males. Incidence peaked before 24 years of age in females and between 25 and 29 years of age among males. The overall prevalence of AGWs based on retrospective administrative databases or medical chart reviews or prospectively collected physician reports Patel et al [3]. Between 379,330 and 510,492 new cases of genital warts in women and between 376,608 and 427,720 in men are estimated in Europe every year [4].
Only in US 14 million persons are infected for first time annually, and 79 million persons have prevalent infection with HPV reports the Review of the Evidence for the 2015 Centers for Disease (CDC) in their Guidelines [1]. Disease expression of HPV includes common warts (verruca vulgaris) which occur in about 20% of school-aged children with prevalence declining with age [5]. But during the years 2013-2014, HPV was reported in nearly half (42.5%) of adults in the United States aged 18–59 years [6]. In men who have sex with men (MSM), a prevalence of HPV infection at the anogenital level has been estimated of 63.9% (37.2% for those at high risk), while in MSM with HIV infection it is of 92.6% (73.5% for those at high risk). At the oral level, the prevalence of infection in MSM is 17.3% (9.2% for high risk) and in MSM with HIV infection it is 27.8% (11.1% for high risk) [7].
While a majority of infections are asymptomatic or self-limited, acquisition of specific types of HPV can result in a significant economic burden in 2010 with 418 million dollars of estimated direct medical costs for non-cervical HPV types related conditions in the US [9] Health problems at US related to HPV include genital warts and cervical cancer.
Genital warts
About 360,000 people in the United States get it each year. More than 10,000 women in the United States get cervical cancer each year. Only in US each year, about 21,000 HPV-related cancers could be prevented with the HPV vaccine [10].
El Quinto Informe Estado de la Región Centro Americana en Desarrollo Humano, 2016, (Fifth Report of the state of Central American Human Development) financed by the International Labor Organization, a United Nations agency, reports that Central America had 9.2 million young people between 15 and 24 years of age in 2016 [13]. Five percent of these that mean 460 thousand individuals will develop genital warts at some point [18].
Despite the worldwide effort to immunize adolescents against HPV (144 million doses of the quadrivalent vaccine vs 41 million doses of the bivalent vaccine), millions of adolescents are infected each year, constituting a vulnerable and high-risk group for anal or genital warts [14].
Thus far, the major clinical focus has been on women, following the recommendations of the World Health Organization (WHO), directing efforts in the prevention of cancer of the uterine cervix; it causes approximately 300,000 deaths worldwide. Ninety five percent (95%) of cervical cancers are due to the high prevalence of high-risk genotypes of human papillomavirus. In fact, the coexistence of genital warts caused by low-risk HPV genotypes is associated up to 50% with those at high risk of producing cancer not only at the female genital level, but at 9 different sites including oral cavity, upper respiratory tract, anus and penis [7,16,17]. It is therefore necessary to recognize genital warts as risk factor for penile or female genital cancer. Hence the importance of this triple-blind randomized clinical trial testing the efficacy of 0.2% gel soluble beta-glucan against 5% imiquimod that is a gold standard treatment in patients of both sexes between 18 and 50 years with genital warts.
Given that cervical cancer is the fourth most common cancer in women worldwide. The World Health Organization (WHO) proposed the goal for the 2021-2030 decade: to accelerate the elimination of cervical cancer as a public health problem through the Global Strategy 90-70-90 launched in November 2020. The truth is that it continues to be cataloged as "one of the most serious threats to women's lives" if prevention, protection and follow-up are not applied at the primary, secondary and tertiary level as proposed by WHO in its Vital Strategy [15].
1. Vaccination of 90% of adolescents globally. As primary prevention, it requires the introduction of the HPV vaccine in all countries to avoid infection, in order to achieve 90% coverage in all women before reaching 15 years of age (9 to 14 years), with a complete vaccination schedule. Considering that the percentage of introduction of this vaccine in WHO Member States is currently 55% and that the average coverage of HPV vaccination is only 54%, considerable investments will be required in the next 10 years to introduce the vaccine in low- and middle-income countries, as well as program improvements to achieve 90% coverage.
2. Secondary prevention consisting of early detection of precancerous lesions and cancer in early stages. This strategy aims for 70% of women be tested with a high precision test between age 30 and 35 and again once before 45 years of age.
3. Tertiary prevention aiming to achieve a good diagnosis, adequate treatment and correct follow-up that increase survival and quality of life for women with cervical cancer, including access to palliative care. The goal is for 90% of women with precancerous lesions and invasive cancers to have access to adequate treatment, monitoring and follow-up.
What are Beta Glucans?
Β-glucans are starch-like glucose polysaccharides except for having β1-3 and β1-6 bonds, instead of the known α 1-4. Its pharmacological properties were discovered hundreds of years ago in oriental medicine working in fungi and researchers in Western medicine 160 years ago. Beta-glucans are chains of D-glucose polysaccharides, linked by beta-type glucosidic bonds. These six-sided D-glucose rings can be connected to each other, at a variety of positions in the D-glucose ring structure.
The process to solubilize the beta-glucan produced in Costa Rica by Dr. Apestegui Barzuna and collaborators in the last ten years, allowed us to take the technological step to optimize the increase in the intracellular action of macrophages and natural killer cells (NK) for various bacterial and viral infections including the human papillomavirus. In addition, it presents a promising future for subsequent antineoplastic therapies pending clinical trials.
The efficacy of beta-glucan in immunological activity was evaluated by Professor Vaclav Vetvicka , PhD, at the Department of Pathology and Laboratory Medicine, University of Louisville, Louisville, Kentucky [19]. Dr. Paola Scardamaglia, et al. were the first to use betaglucanes topic in cervix uterine in 2010 as treatment of NIC in patients with very good results. She could demonstrate the effectiveness of the treatment with beta-glucan in the women with ASCUS-LSIL lesions and HPV-CIN1 lesions, increasing of the regressions rate after 12 months of the treatment of the 15-20% [21].
Methodological Design
Type of study randomized triple-blind clinical trial.
Study area
The Saint Dominic Clinic in the department of Managua, Nicaragua Study population: All participants with anogenital warts who present to the Saint Dominic Clinic in Managua.
Sample
One hundred two individuals, active patients at the Saint Dominic Clinic, Managua, Nicaragua, who meet the inclusion criteria of having anogenital warts diagnosed by Polymerase Chain Reaction (PCR) or Hybrid Uptake-2 in papillomavirus are; 50% women and 50% men. As of the date of this report we have recruited 36 cases for the Clinical Trial Study Type: Triple-blind randomized clinical trial.
The intervention group (Group A) will receive 0.2% beta-glucan gel and the control group (Group B) will receive 5% imiquimod cream, which is the reference treatment with a clearance rate of 51%.
The sample size was calculated assuming:
-Clearance after beta-glucan treatment p1 = 80% and
-Clearance after treatment with Imiquimod 5%: p2 = 51% [ 20].
The formula is as follows
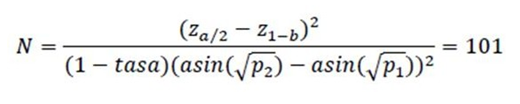
• Power of b = 80%
• Confidence level of = 95%
• Drop rate = 20%
Exclusion criteria:
• Pregnant women, puerperal women, lactating women and with positive cervical intraepithelial neoplasia (CIN).
• Vaccinated against Human Papilloma Virus (HPV) (Gardasil, Gardasil 9 or Cervarix)
•Immunosuppressed by drugs (corticosteroids, prolonged chemotherapy or antibiotic therapy, anti-pulmonary tuberculosis treatment) or taking natural or synthetic immunomodulatory products.
• Diagnosed with any of the following: molluscum contagiosum, Botryoid sarcoma, Herpes type II
• Having skin appendages
• With urethral prolapse
• History of surgery in the genito-anal area in the last 4 weeks
1. PROF. DR. VACLAV VETVICKA, PH.D. World's leading expert on betaglucanes, University v Louisville, Kentucky, USA Professional CV. Vaclav Vetvička, Ph.D., is Professor of Pathology in the Department of Pathology of the University of Louisville in Louisville, KY. He graduated from Charles University in Prague, Czech Republic, with an advanced degree in biology and obtained his Ph.D. from Czechoslovak Academy of Sciences. His postgraduate training included a stay at the Oklahoma Medical Research Foundation, Oklahoma City, and at the Institute of Microbiology, Prague, Czech Republic. His main area of research interest focuses on the development of natural immunomodulators. In addition to the glucan research, Dr. Vetvicka helped to launch glucan in several countries including USA, France, Turkey and Czech Republic. Dr. Vetvicka is author and co- author of more than 190 peer-reviewed publications, seven books and five international patents.
2. Dr. Scardamaglia et al. reports after 3 months of treatment, of the 30 women with positive cytology and negative colposcopy, 80% with ASCUS diagnosis resulted negative, 35% with LSIL diagnosis resulted negative; after 6 months 100% with ASCUS diagnosis resulted negative, 70% with LSIL diagnosis resulted negative; after 12 months 85% with LSIL diagnosis resulted negative. Of the 30 women with positive cytology, positive colposcopy and HPV-CIN1 histology after 3 months 20% resulted negative, after 6 months 60% resulted negative and after 12 months 80% resulted negative. The persistence of the HPV-CIN1 histology was verified in the 13% of the women. For these women the definitive treatment was the TFD.
Processing and production of soluble beta-glucan
The beta-glucan applied in 0.2% gel, in this clinical trial, is obtained from the bread yeast (Saccharomyces cerevisiae) through a process of digestion. The proteins and nucleic acids are removed along with other polysaccharides and sugars. Once purified, it is washed with purified water to remove residues and subjected to a process of solubilization to improve molecular bioactivity.
Investigation report - Preliminary findings
We have advanced 35% of the sample proposed for analysis, we have the following preliminary findings:
This far we have recruited 36 participating; 20 men and 16 women, with an average age of 26 years old. In branch “A” we have 20 participants and in branch “B” 16 participants. As stated before, due to the COVID 19 epidemic the clinical trial was stopped on August 2019.
• More than 70% of participants have had at least 5 sexual partners and practice 90% oral sex
• Pink or gray genital warts are of greater therapeutic response with beta- glucan gel, especially those located on the glans penis and vagina.
• The clearance rate in branch A with beta-glucan is 80%. Meanwhile the clearance rate in branch B with imiquimod is 40% up to the time of this report.
A diabetic male with extensive gland lesions in cauliflower responded very well to beta-glucan. We detected 3 squamous atypia on the cervix.
With the hybrid uptake type 2 test we detected the coexistence of high-risk strains of HPV with genital warts in 40% of the 36 cases.
In conclusion, it is necessary to complete the clinical trial for a valid comparison of soluble Beta-Glucan at 0.2% vs. the actual gold standard of Imiquimod at 5% [18].
Acknowledgement
This research was carried out within the 2019 work plan of the International Center for Medical Research Dr. Italo Fabbri (Spanish- Centro Internacional de Investigaciones Médicas Doctor Italo Fabbri) of which Dr. Perez Fabbri A. is the executive director. Special thanks to Dr. Angel Rafael Braña-López, board certified specialist in preventive medicine from Puerto Rico, for his methodological contribution in translating this report.
Conflict of Interest Statement
Corporate sponsors: Laboratorio Calox of Costa Rica, S.A., Laboratories Piersan Centro Americana S.A. of Guatemala. Institutional sponsor: Nuevo Hospital Monte España of Nicaragua through its Medical Director Dr. Silvia Guerrero, gynecologist and oncologist in the processing of the Hybrid Captation type 2 or PCR test for HPV.
References
1. Park u Ina, Introcaso Camille, Dunne F Eileen. Human Papillomavirus and Genital Warts: A Review of the Evidence for the 2015 Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis. 2015;61: S849-55. Doi: 10.1093/cid/civ813.
2. Pagliusi S. Vaccines against Human Papillomavirus. World Health Organization; Retrieved January 25, 2009 from the World Wide Web: http://www.who.int/vaccines/en/hpvrd.shtml.
3. Patel H. Systematic review of the incidence and prevalence of genital warts. BMC Infect Dis. 2013; 13:39.
4. Buckley B. Anogenital warts: incidence, prevalence, self-reported history and quality of life. Cochrane Response, London, UK. 2016. Available from: http://www.who.int/immunization/sage/meetings/2016/october/03_Burden_of_anogenital _warts.pdf.
5. Kilkenny M, Merlin K, Young R, et al. The prevalence of common skin conditions in Australian school students:1. Common, plane and plantar viral warts. Br J Dermatol. 1998; 138:840-5.
6. Satterwhite CL, Torrone E, Meites E, et al. 2013. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sex Transm Dis. 2013; 40:187-93.
7. Elena Sendagorta-Cudós, Joaquín Burgos-Cibrián, Manuel Rodríguez-Iglesias. Genital infections due to the human papillomavirus. DOI: 10.1016/j.eimc.2019.01.010 https://www.elsevier.es/es-revista-enfermedades-infecciosas-microbiologia-clinica-28-articulo-infecciones-genitales-por-el- virus-S0213005X19301223
8. Reporte anual sobre infecciones de transmisión sexual 2018. OMS. http://www.who.int/es/news-room/fact-sheets/detail/sexually-transmitted-infections.
9. Vivian Colón-López, Ana P. Ortiz, Joel Palefsky. Burden of Human Papillomavirus Infection and Related Comorbidities in Men: Implications for Research, Disease Prevention and Health Promotion among Hispanic Men Published in final edited form as: P R Health Sci J. 2010; 29(3): 232–240.
10. CDC 2017 Infección genital por VPH: Hoja informativa https://www.cdc.gov/std/spanish/vph/stdfact-hpv-s.htm Last revision: Feb 07, 2017. Fuente del contenido: División para la Prevención de Enfermedades de Transmisión Sexual, Centro Nacional para la Prevención de VIH/SIDA, Hepatitis Virales, ETS y Tuberculosis.
11. S. de SanJosé FX, Bosch y X. Castell Sagué Instituto Català de’Oncologia. Epidemiologia de la infección por virus del papiloma humano y cáncer de cervix L’ Hospital et de Llobregat. Barcelona SEMERGEN. 2007; 33Supl 2:9-21
12. Palacios S. Cuantas personas tienen el virus del papiloma humano? October, 2019. https://institutopalacios.com/cuantas-personas-tienen-el-virus-del-papiloma-humano-vph-hpv/
13. Quinto Informe Estado de la Región.03/08/2016. https://www.bcie.org/novedades/eventos/evento/quinto-infome-estado-de-la-region-2016
14.Neyro JL. 2014. https://www.neyro.com/2014/05/19/la-comision-europea-ha-aprobado-gardasil-la- vacuna-cuadrivalente-para-prevenir-el-virus-del-papiloma-humano-vph/
15. Pan American Health Organization PAHO 2020. A Global Strategy for elimination of cervical cancer https://www.paho.org/en/towards- healthier-generations-free-diseases/global-strategy-elimination-cervical-cancer
16. H zur Hausen, et al. Papillomavirus infections--a major cause of human cancers. Biochim Biophys Acta. 1996;1288(2):F55-78. doi: 10.1016/0304-419x(96)00020- 0. MID: 8876633 https://pubmed.ncbi.nlm.nih.gov/8876633/
17. Laura Daniela Sierra-Bossa, Julián Ricardo Zapata-Rozo, Diego Alejandro Rangel-Rivera. Malignant progression of anogenital condyloma acuminata associated with low-risk Human Papillomavirus in women. Medicas UIS. 2020; 33: 1. https://doi.org/10.18273/revmed.v33n1-2020001. Hospital Universitario de Santander. Profesor Cátedra. Universidad Industrial de Santander. Bucaramanga, Colombia. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0121-03192020000100009
18. Baker A. David, Ferris Darron G, Martens Marc, et al. Infectious Disease in Obstetrics and Gynecology, Vol. 2011, Article ID 806105,11 pages. Imiquimod 3.75% Cream Applied Daily to Treat Anogenital Warts: Combined Results from Women in Two Randomized, Placebo-Controlled Studies Pub, August 2011.
19. Vetvicka V. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Tri Can Fam Physician. Molecules. 2013;59(7): 731-6.als. 2019 http://glucan.us/
20. Lopaschuk Catharine, et al. New approach to managing genital warts Review Can Fam Physician. 2013;59(7):731-6. PMID: 23851535 PMCID: PMC3710035. https://pubmed.ncbi.nlm.nih.gov/23851535/
21. P Scardamaglia , C Carraro, P Mancino, et al. Effectiveness of the treatment with beta-glucan in the HPV-CIN 1 lesions. [Article in Italian]. Affiliations expand Minerva Ginecol. 2010;62(5):389-93. PMID: 20938424
Received: October 11, 2021;
Accepted: October 25, 2021;
Published: October 28, 2021.
To cite this article : Fabbri AP, Álvaro Apestegui Barzuna AA, Fantin R. Betaglucan 0.2% versus Imiquimod 5% in the Treatment of Ano Genital Warts (Agw). Health Education and Public Health. 2021; 4:3.
© 2021 Fabbri AP, et al.
